Influenza Viral Testing
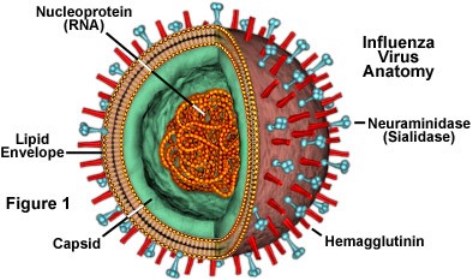
Influenza A and B viruses are responsible for respiratory epidemics each year. Influenza A Viruses are categorized by their surface proteins: Hemagglutinin (H) and Neuraminidase (N). Since Influenza B viruses are structurally different, they are only broken down into two lineages: B/Yamagata and B/Victoria (CDC, 2017).
The Delaware Public Health Lab performs influenza identification, molecular detection, and subtyping. Influenza surveillance is performed as part of the CDC-World Health Organization (WHO) Influenza surveillance effort. This information, along with a select number of isolates, is provided to CDC and the WHO to aid in surveillance efforts and future vaccine formulation.
What We Do
- Real-Time Reverse Transcriptase Polymerase Chain Reaction (rRT-PCR)
- Influenza SARS-CoV-2 (Flu SC2) Multiplex rRT-PCR
- Influenza A subtyping by rRT-PCR
- Influenza B genotyping
- Seasonal H3N2 detection
- 2009/H1N1 Pandemic detection
- H5N1 Avian (presumptive result)
- H3N2 variant (presumptive result)
- H7N9 (presumptive result)
- Pyrosequencing – for anti-viral resistance on a subset of A/H3N2 and pandemic A/H1N1 influenza specimens
- Respiratory Virus Panel (RVP) – For outbreaks, fatalities, or specimens (with prior approval from the Office of Infectious Disease Epidemiology
Acceptable Specimens
- Nasopharyngeal washes, nasopharyngeal aspirates, nasopharyngeal swabs
- nasal or throat swabs, dual throat/nasal swabs
- bronchial washes, bronchoalveolar lavage
- lung tissue (this includes lung biopsy, bronchial biopsy, and postmortem lung specimens)
- sputum
- viral culture isolates
- Collection Kit here: HerpesViralFluPertussis Collection kit-Instructions
Submitting a Specimen
- Obtain collection kits from the DPHL. This kit will contain a tube with viral transport media and two dry NP swabs, and the Request for Clinical Testing form.
- Fill out the form completely.
- Label the specimen with name of patient and date of birth (or barcode, if applicable).
- Arrange for specimen transport to the laboratory.
DPH Flu Immunization Information
Delaware Weekly Influenza Surveillance Reports
CDC Guidelines for Clinicians on the Use of Rapid Influenza Diagnostic Tests
References: Centers for Disease Control and Prevention, 2017. Types of Influenza Viruses.
Return to the Delaware Public Health Laboratory page
This page was last updated 4/2024
![]() Please note: Some of the files available on this page are in Adobe PDF format which requires Adobe Acrobat Reader. A free copy of Adobe Acrobat Reader can be downloaded directly from Adobe . If you are using an assistive technology unable to read Adobe PDF, please either view the corresponding text only version (if available) or visit Adobe’s Accessibility Tools page.
Please note: Some of the files available on this page are in Adobe PDF format which requires Adobe Acrobat Reader. A free copy of Adobe Acrobat Reader can be downloaded directly from Adobe . If you are using an assistive technology unable to read Adobe PDF, please either view the corresponding text only version (if available) or visit Adobe’s Accessibility Tools page.



