Current Suspected Overdose Deaths in Delaware for 2025: Get Help Now!
Find school water testing results and additional resources
Attention Medicaid Participants: Eligibility Renewals Restarted April 1, 2023
The recommendations in this Health Alert are based on the current situation and knowledge. Recommendations are changing quickly and this Health Alert will be updated as necessary.
The Delaware Division of Public Health (DPH) is reporting 44 confirmed cases of swine-origin influenza A (H1N1) virus infection. The onset of the first case was April 24. In addition, more than 500 symptomatic University of Delaware Students were treated for antiviral agents at the University Health Center or a Division of Public Health clinic between April 29 and May 1.
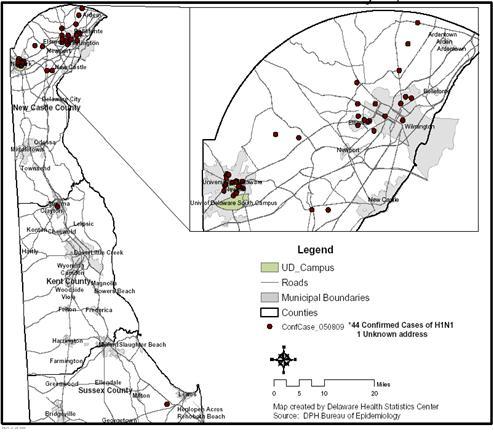
The median age of confirmed cases is 19 years. 59% (26) of the cases are male. 55% (24) of the cases are known University of Delaware students. Only 2 cases have a known history of travel to Mexico. The epidemic curve and geographic distribution are below (Figures 1 and 2).
Figure 1 Date of Onset of Confirmed Cases of H1N1 Influenza, Delaware, Through May 8, 2009
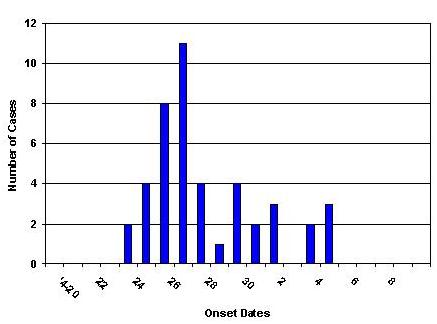
Figure 2 Confirmed Cases of H1N1 Influenza, Delaware, Through May 8, 2009
In addition to monitoring the epidemic in Delaware through laboratory confirmation of cases, DPH has implemented enhanced surveillance of ILI. While not all components of enhanced surveillance are fully in place, they include:
Not all people with suspected novel influenza (H1N1) infection need to have the diagnosis confirmed. Patients with symptoms that are not severe can be treated empirically (see “Treatment” below).
Clinicians should test persons for the novel influenza (H1N1) virus if they have an acute febrile respiratory illness or sepsis-like syndrome. Certain groups may have atypical presentations including infants, elderly and persons with compromised immune systems. Priority for testing includes persons who 1) require hospitalization or 2) are at high-risk** for severe disease. To test for novel H1N1 influenza virus, upper respiratory specimens, such as a nasopharyngeal swab or aspirate, nasal swab plus a throat swab or nasal wash, or tracheal aspirate should be collected. Persons who perform nasal and tracheal aspirate collections on ill persons require appropriate personal protective equipment.
For the purposes of disease surveillance only, DPH is seeking specimens from persons other than University of Delaware students with influenza-like symptoms if they present with a fever >100° F and cough, sore throat, or nasal congestion.
Specimen storage and transportation information can be found here: http://www.dhss.delaware.gov/dhss/dph/php/alerts/dhan177.html
Treatment is recommended for:
If a patient is not in a high-risk group or is not hospitalized, healthcare providers should use clinical judgment to guide treatment decisions, and when evaluating children should be aware that the risk for severe complications from seasonal influenza among children younger than 5 years old is highest among children younger than 2 years old. Many patients who have had novel influenza (H1N1) virus infection, but who are not in a high-risk group have had a self-limited respiratory illness similar to typical seasonal influenza. For most of these patients, the benefits of using antivirals may be modest, with efficacy decreasing significantly when administered past 48 hours of onset of symptoms. Therefore, testing, treatment and chemoprophylaxis efforts should be directed primarily at persons who are hospitalized or at higher risk for influenza complications.
Post exposure antiviral chemoprophylaxis with either oseltamivir or zanamivir can be considered for the following:
Pre-exposure antiviral chemoprophylaxis should only be used in limited circumstances. Certain persons at ongoing occupational risk for exposure who are also at higher risk** for complications of influenza (e.g., health care personnel, public health workers, or first responders who are working in communities with influenza A H1N1 outbreaks) should carefully follow guidelines for appropriate personal protective equipment or consider temporary reassignment.
* Close contact, for the purposes of this document, is defined as having cared for or lived with a person who is a confirmed, probable or suspected case of novel influenza A (H1N1), or having been in a setting where there was a high likelihood of contact with respiratory droplets and/or body fluids of such a person. Examples of close contact include kissing or embracing, sharing eating or drinking utensils, physical examination, or any other contact between personslikely to result in exposure to respiratory droplets. Close contact typically does not include activities such as walking by an infected person or sitting across from a symptomatic patient in a waiting room or office.
** High-risk groups: A person who is at high-risk for complications of novel influenza (H1N1) virus infection is defined as the same for seasonal influenza at this time. As more epidemiologic and clinical data become available, these risk groups might be revised.
For further information contact the Delaware Division of Public Health at (302) 744-4700.